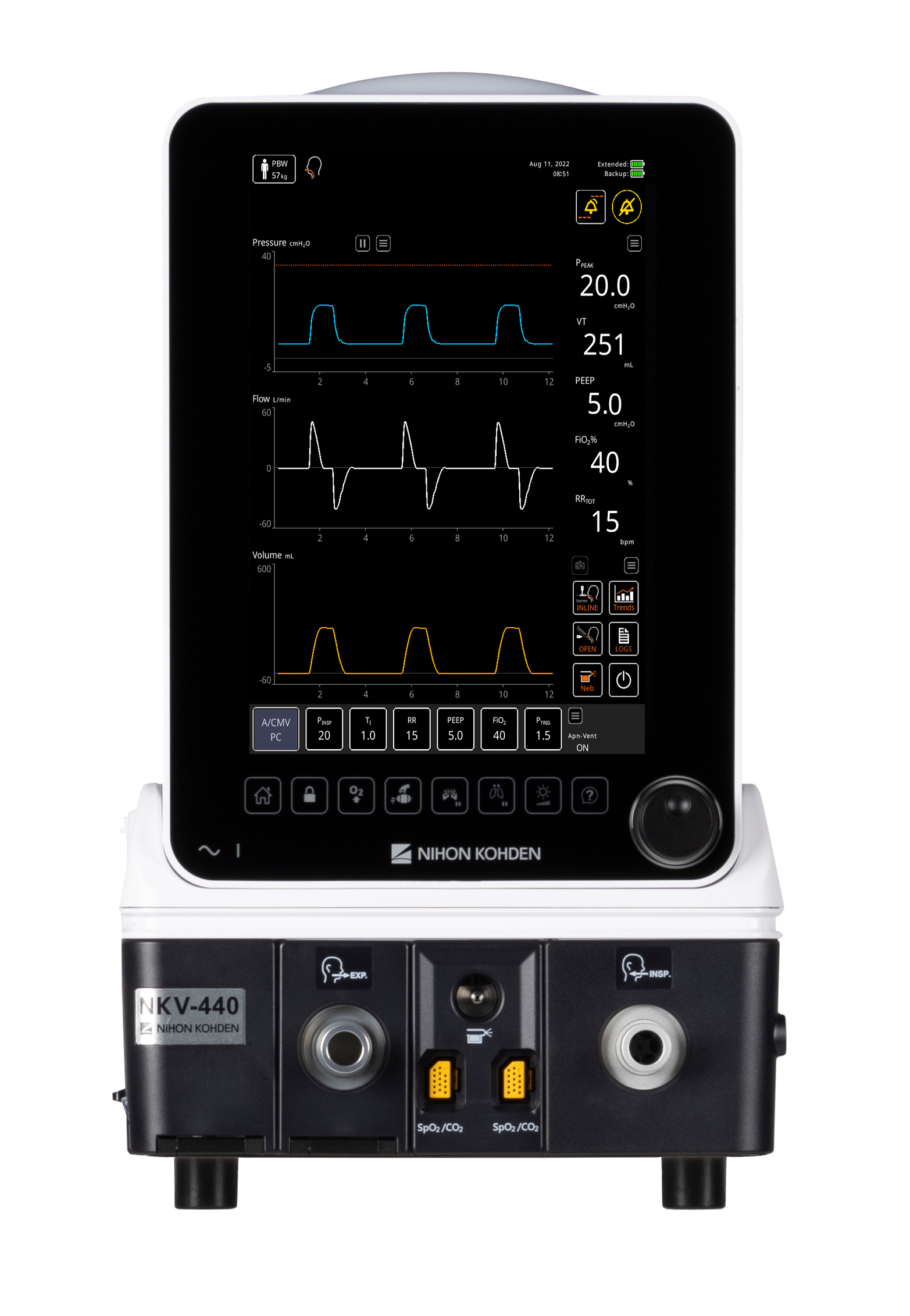
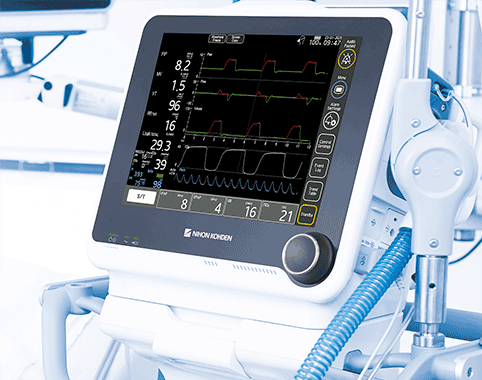
19 Jun Nihon Kohden NKV-440 Ventilator System Receives US FDA 510(k) Clearance
Today, Nihon Kohden OrangeMed, LLC received U.S. Food and Drug Administration (FDA) 510(k) clearance for its NKV-440 Ventilator System. The NKV-440, an turbine-based ventilator, is a “neonatal through adult” platform that provides independence from compressed air while providing high-quality ICU performance in a smaller, more...